The COVID-19 vaccine is now available to children ages 5 to 11. Here’s what parents and children need to know about the safety and effectiveness of the vaccine, the possible side effects, and the benefits of getting vaccinated.
We Recommend the COVID-19 Vaccine for Children Age 5-11
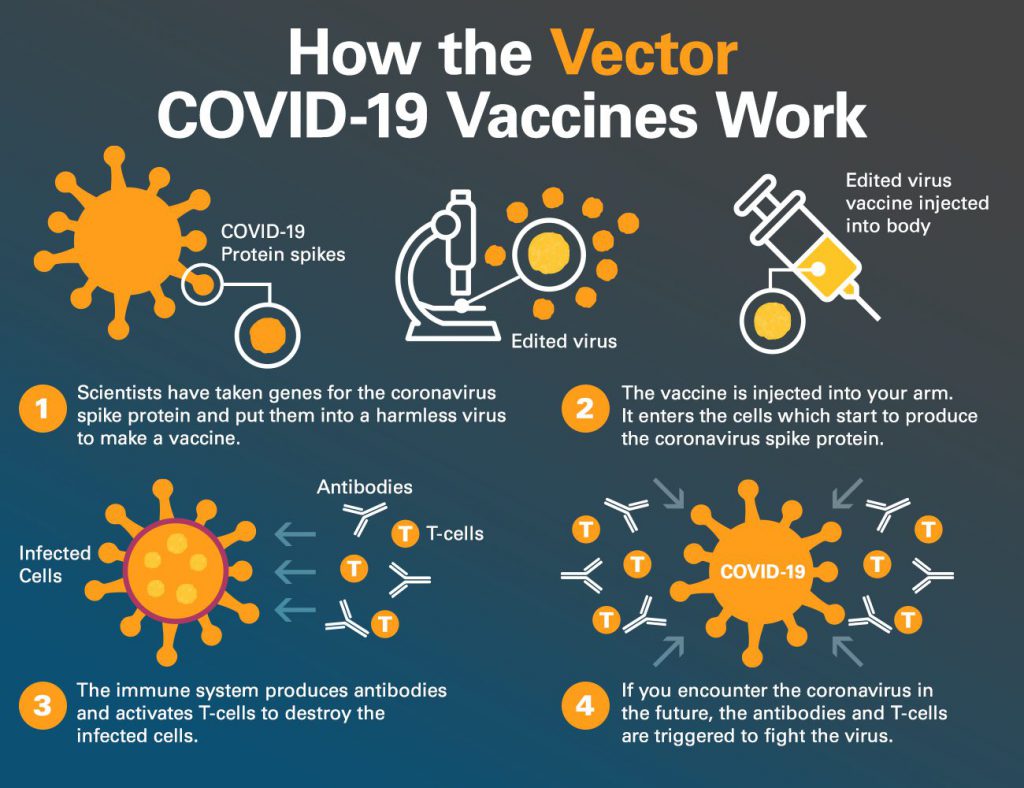
On November 2, 2021, the Pfizer COVID-19 vaccine was authorized for emergency use for children ages 5-11. It is a 2-dose series taken three weeks apart. Each dose will be 1/3 the dosage of the adolescent/adult vaccine.
The vaccine for children ages 5-11 years is effective:
· 90-100% effective in clinical trials.
· There were no severe cases of COVID-19 in clinical trials.
· The vaccine works against Delta and other known variants of concern.
IHA Pediatrics has been vaccinating children ages 12 and up since May of 2021, and we have confidence that the Pfizer COVID-19 vaccine is safe and effective for our patients. As of October 2021, more than 11.1 million adolescents have been vaccinated against COVID-19 with the Pfizer COVID-19 vaccine.
Vaccine Appointment Available
IHA Pediatrics is now offering COVID-19 vaccines to current patients aged 5-11 however, availability may be limited.
We are working quickly to begin vaccinating our younger patients and will share more information on how to schedule your child’s COVID-19 vaccine in the coming days. Please continue to check our website and social media for the latest updates. If your child has an appointment scheduled at IHA Pediatrics in the next few weeks and is eligible for the COVID-19 vaccine, please ask for it at your appointment.
We also encourage you to search vaccines.gov to enter your zip code and easily find locations where vaccines are available.
Don’t Forget the Flu Vaccine!
It is more important than ever to stay healthy and protect yourself against preventable illnesses. It is safe to receive the flu vaccine at the same time as the COVID-19 vaccine.
Top Ten Questions Parents Ask about the COVID-19 Vaccine
Should I worry the vaccine is too “new”? No.
As of October 2021, more than 6.63 billion doses of the COVID-19 vaccine have been given worldwide, with more than 416 million doses in the United States. For adolescents alone, over 11 million doses of the COVID-19 vaccine tell us that this vaccine is no longer “new.” Scientists and pediatricians feel confident in the safety of the COVID-19 vaccine. Waiting puts you at higher risk for infection and illness.
The vaccine got to us fast due to:
- The vaccine research for mRNA started in 1961 and, in the last decade specifically, was focused on SARS.
- The vaccine was released more quickly than other vaccines because the production started before the clinical trials. This was due to the pandemic, which provided funding and resources to make that happen.
- Due to high disease rates in our community during vaccine development, we didn’t have to wait for a minimum number of cases for clinical trials, as is standard with vaccine development.
Will we need booster shots every year? We don’t know yet.
It depends on how many people get vaccinated and if the virus continues to spread and change. As the population becomes vaccinated, we reduce the spread of the virus, which helps to prevent it from continuing to change. We won’t need boosters if we are reducing and eliminating variants of COVID-19.
Does it affect puberty or fertility? No.
Based on our knowledge of mRNA, we are confident that the COVID-19 vaccine will not have long-term effects on puberty or fertility. mRNA cannot integrate with DNA or alter cells.
- Vaccine ingredients are cleared from the body quickly. mRNA is fragile and breaks down within 72 hours after injection. Ingredients do not linger in the body.
- Thousands upon thousands have gotten pregnant after receiving the COVID-19 vaccine.
- mRNA vaccine is not made up of COVID-19. It is only the protein.
- There are reports of menstrual cycle changes after the COVID-19 vaccine. This is due to the body mounting an immune response and a temporary side effect, like a fever.
What are the most common side effects for children? They can vary but are minimal.
- Side effects that have been reported are mild to moderate such as fever, fatigue, headache, chills, diarrhea, or muscle aches.
- More children reported side effects with the second dose compared to the first dose.
- Rare side effects can happen, such as swollen lymph nodes or skin sensitivity, but these are not long-term and resolved in most cases in a few days.
How do we know about long-term side effects? Decades of research.
Based on our knowledge of mRNA and the human body, we don’t expect long-term side effects since it breaks down in the body in 72 hours.
- As with all vaccines, including the COVID-19 mRNA vaccines, concerning side effects have occurred 6-8 weeks after injection. Vaccine development is based on decades of research. Scientists have done a rigorous review of all available data before approving for children. Our history of science tells us that if there are no side effects in those first few weeks, we are confident that concerns that arise with any patient decades later are unlikely to be related to any vaccine.
- mRNA cannot be converted to or inserted into DNA. It’s not scientifically possible.
How common is myocarditis for children after vaccination? Extremely rare.
Myocarditis means “inflammation of the heart muscle.” This can happen due to the robust immune response the vaccine can have on your body.
- It is very rare, about 26 cases per 1 million.
- Myocarditis has occurred rarely in some people following the mRNA COVID-19 vaccines, typically within a week after the second dose.
- The risk is highest in males 12–29 years of age.
- The risk of myocarditis after the mRNA COVID-19 vaccine is lower than the risk of myocarditis from the actual COVID-19 virus in adolescents and adults.
- When myocarditis occurs in those with COVID-19 infection, it is more common, severe, and lasts long-term.
- No cases of myocarditis were reported in the vaccine clinical trial among ~3000 children, ages 5–11 years.
- Symptoms of myocarditis are most commonly chest pain, difficulty breathing, or a fluttering heartbeat.
- Adolescents who have had this rare side effect are monitored closely. Most make a full recovery in 3-4 weeks by using anti-inflammatory medications like ibuprofen.
- No children have died of myocarditis after the COVID-19 vaccine.
My child had COVID. Do they need the vaccine? Yes.
- We know that “natural immunity” can be high at first. However, protection can drop off quickly or change based on circulating variants.
- Getting a vaccine, even for those who have already had COVID-19, strengthens your immune response.
- If you had COVID-19 once, it is possible to get a different strain again. The immune response after infection is not as focused. Evidence shows the vaccines protect you longer and for all the variants to date.
- Most importantly, the vaccine gives protection and prevents hospitalization for several of the COVID variants.
- Your child can get the COVID-19 vaccine once they are out of quarantine. There is no “waiting period,” as another strain may come, and the vaccine will protect from getting hospitalized.
Can children become very sick with COVID? Yes.
COVID-19 disease in children can range from no symptoms to severe illness.
- As of October 2021, over 6.3 million COVID-19 pediatric cases have been reported.
- Only 43% of children under 12 have natural immunity.
- 30% of hospitalizations for children with COVID-19 had no underlying medical conditions. As of October 2021, there were 5,217 MIS-C cases linked to COVID-19 in children. This multi-organ system effect makes children extremely ill and requires hospitalization, often in the ICU.
- Long COVID, or lingering COVID-19 symptoms, can lead to learning problems, heart problems, exercise fatigue with sports, and respiratory issues. This has been reported in about 8% of children who have had COVID-19.
- Since the pandemic began, over 600 pediatric deaths due to COVID-19 have been reported. It is now a top 10 cause of death for children in the United States.
What are the ingredients? Put simply, it’s fat, salt, electrolytes, and sugar.
- Lipids are the “fatty layer” that protects the delicate mRNA so it has time to work before getting chopped up. Polyethylene glycol (PEG), the most famous lipid, is also the main ingredient in MiraLAX (which you know about if your child has ever been constipated).
- The ingredients are only just salt, some electrolytes, mRNA, fat, and sugar. These ingredients help keep the vaccine stable and are natural preservatives.
Is there less quarantine from school, sports, or activities if vaccinated? Yes.
- This pandemic has been traumatizing, especially for children. Their lives were abruptly disrupted in March 2020, and their mental and physical health has suffered. Anxiety and depression rates are up.
- Based on the State of Michigan’s current guidelines, students who are vaccinated and exposed to COVID-19 can remain in school and wear a mask.
- We know that less quarantining will only benefit all children.